Two teams from Pohang University of Science and Technology have collaborated to develop a new catalysis agent used in the production of hydrogen. They have also identified a new correlation in hydrogen catalyst properties that will aid future development of efficient catalysts.
Hydrogen fuel is a zero-carbon fuel that burns cleanly to produce only energy and water. It is being touted as an important part of humanity’s future transition away from fossil fuels. As it stands however, around 95% of hydrogen is produced from fossil fuels, emphasising how important the research of alternative production pathways is.
The research has focused on sourcing a cheap way to carry out the hydrogen evolution reaction (HER) – a chemical process in which water is split apart in the presence of a catalyst to produce hydrogen fuel (H2). The most widely used catalysts currently in use are platinum-based in an acidic environment, with the high price of platinum making it economically unviable on a large scale.
The issue facing scientists is that inexpensive catalysts are only stable under alkaline conditions, in which the HER reaction is between 10 and 100 times slower. This is what the team at Pohang University sought to tackle.
Catalysts aid in the reaction through a process known as adsorption. This is where metals with certain properties temporarily bond with elements to ‘hold them in place’ to speed up reactions. In the normal HER reaction, under acidic conditions, hydrogen ions are readily available to bond together.
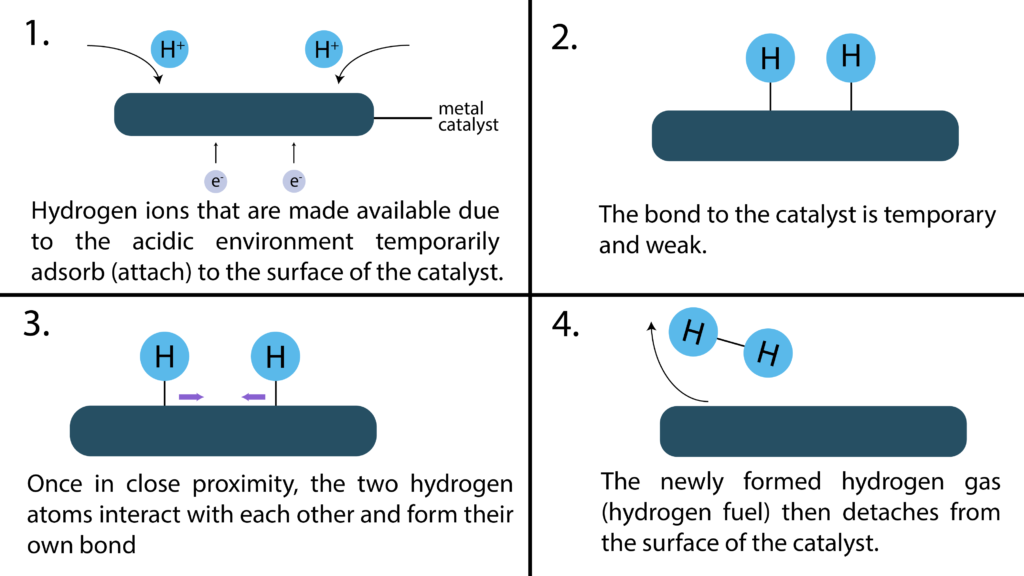
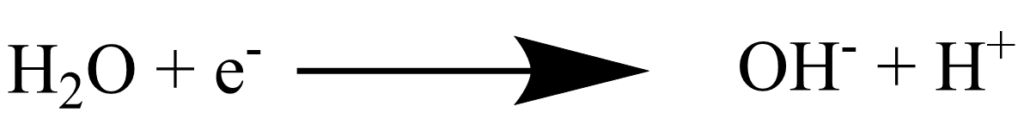
In alkaline conditions however, there is an extra dissociation step. This is the cause of the slower reaction rates.
By including secondary elements, other teams have successfully sped up the alkaline based reactions, however many have still relied on expensive metals. Therefore, a greater understanding of the relationship between the catalyst’s structures and their effectiveness is needed to develop more viable catalysts.
The team at Pohang University went about testing a range of ‘oxophilic’ metals – ones that bound OH– ions. This was based of previous studies that have shown increased adsorption of OH– ions increased the effectiveness of the catalyst.
They found that the OH– binding energy directly affected the reaction energy and the speed of overall hydrogen evolution.
A team of chemical engineers led by Professor Jeong Woo Han then developed a nickel nanohelix doped with chromium, based off the scientific principles demonstrated by the other team.
Chromium was chosen because it enhanced both OH– binding and H+ binding. Nickel was chosen because the structure of the catalyst provided many “active sites” – areas where the reaction can occur.
According to the paper, the nanohelix catalyst displayed “remarkably enhanced catalytic activities”.
Professor Jong Kyu Kim, corresponding author of the paper said, “This research is significant in that it provides the scholarly foundation for high performance and commercialization of sustainable hydrogen energy conversion system,” adding that “this original technology will have significant ripple effects and technological expansion in the environmental energy sector.”
As humanity moves away from fossil fuels, there is no doubt that research like this will attract significantly more funding. For now, this stands to be the one of the first steps in economically viable hydrogen fuel production.
